© 2024 All Rights Reserved Enewspolar
TASHKENT, Dec 24 (Xinhua) -- Uzbekistan has placed an order for 2 million doses of COVID-19 vaccine through the COVAX initiative led by the World Health Organization, an Uzbek health official said Tuesday. "Uzbekistan has become a member of COVAX, and our application for 2 million doses was approved," Deputy

NEW DELHI, Dec. 23 (Xinhua) — The recovery rate of COVID-19 patients in India Wednesday increased to 95.68 percent, the
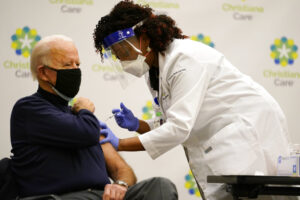
NEWARK, Dec 22 (AP) — President-elect Joe Biden on Monday received his first dose of the coronavirus vaccine on live

AMSTERDAM, Dec 21 (AP) — The European Medicines Agency recommended conditional approval Monday for a coronavirus vaccine developed by BioNTech
China’s COVID-19 vaccines expected to be rolled out soon; bulk manufactured and exported in 2021. China could likely mass inoculate
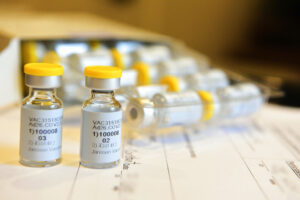
CAIRO, Dec 11 (AP) — Egypt on Thursday received its first shipment of a Chinese coronavirus vaccine, which was tested

By DANICA KIRKA Associated Press LONDON, Dec 08 (AP) — U.K. health authorities are rolling out the first doses of

NEW DELHI, Dec. 08: Two vaccine manufacturers — Pfizer and Serum Institute of India (SII) — have applied for the

BEIJING, Dec 7 (AFP) – Chinese pharmaceutical firm Sinovac Biotech has secured half a billion dollars in extra funding to

JAKARTA, Dec 06 (AP) — Indonesia’s government said 1.2 million doses of a COVID-19 vaccine developed by China-based biopharmaceutical company